Principe
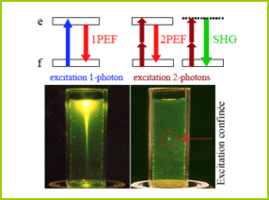
First described by Maria Goeppert-Mayer in 1931, two-photon absorption is the concept that two photons of the same or different frequencies can excite a molecule from one energy state (usually the ground state) to a higher energy state in a single quantum event.
For this to occur, the two photons must strike the molecule within a femtosecond of each other (10-15 seconds). This requires a focused laser capable of producing very fast light pulses (~80 MHz) with high power (~150,000 W peak power).
The wavelength of light is proportional to its energy level. The shorter the wavelength, the more energy it has. This is a linear relationship, so a photon with a wavelength of 400 nm has twice the energy of a photon with a wavelength of 800 nm. Traditional fluorescence microscopy uses a single photon to excite fluorescent probes using primarily visible excitation wavelengths (390-700 nm). After excitation, the electron falls back to its steady state and, in the process, releases a photon of light with slightly less energy than the excitation photon (lost due to processes such as vibrational relaxation).
In two-photon microscopy, two photons of light of double wavelength are used to excite the fluorescent probes. However, only one photon is released when the electron descends into its most stable orbit. This photon will have the same wavelength as the equivalent one-photon fluorescence method (i.e., about half the excitation wavelength). The wavelengths used to excite these same dyes with two photons therefore tend to be between about 800 and 1000 nm, in the infrared spectrum.
One of the main advantages of two-photon microscopy is its ability to limit excitation to a tiny focal volume in thick samples. The focal point of the objective is the only space with a high enough photon density to ensure that two photons are presented to the fluorophore simultaneously. This means that there is no out-of-focus emission light and that all light at the emission wavelength must originate from this single point. In "1-photon" fluorescence microscopy the laser excites the fluorescent probe throughout the sample or through a cone of light to the focal plane (e.g., in confocal microscopy). This leads to greater out-of-focus excitation, resulting in faster photobleaching and significant phototoxicity (the toxic effect of activated fluorophores on cells).
The use of infrared wavelengths has an additional advantage. Light in the visible spectrum scatters greatly in biological tissues, severely limiting the depth to which it can penetrate with sufficient power to excite a fluorophore. Infrared lasers used for two-photon microscopy scatter much less. This means that the infrared laser light has enough power to excite fluorophores down to about 1 mm in living tissue. In comparison, single-photon confocal microscopy can only penetrate to about 200 µm.
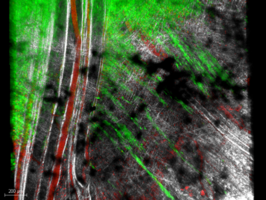
Visualization of certain structures (collagen, fibers, muscles, elastin, laminin...) is also possible without second harmonic (SHG) and third harmonic (THG) marking.
Few applications :
- high resolution imaging on thick tissues (zebrafish, drosophila, explants, transparencies, organoids...)
- Multi-modality imaging (coupling whole body imaging and high resolution organ imaging,...)
- High resolution in vivo imaging on mouse optical chambers
More explanation:
Campus Pasteur
Description :
- Marque : Leica SP8-MP
- Type de statif : Droit motorisé
- Logiciel : LAS X
- Laser continu (nm) : 488 / 552 / 633
- Laser infra rouge pour excitation multiphotonique : Coherent Chameleon VisionII avec Pre-compensation 650-1040nm
- Modalité : Microscope confocal et multi-photon
- TL : BF
- Détecteurs : 1 GaAsP (interne) + 1 PMT | NDD multi-photon 2 HyD (dichroïque GFP525/50-RFP585/40 ou SHG630/10-Cy5685/40)
- Objectifs : 5x/0.15 | 10/0.30 | 25x/0,95 (eau, longue distance 2mm) | 40x/1,1 (eau) | 63x/1.20 (eau) et 20x compatible light-sheet
- Platine : Motorisée
- Chambre d'incubation Temp. et CO2 : Okolab
- Supports : Tout type de montages d’échantillons fixés ou prélèvements ex-vivo / support de contention pour imagerie in-vivo s
- Z-Stacks : Oui
- Mosaïques : Oui
- Multi-positions : Oui
- Maintien de focus : Definite Focus
- Accessoires : Système d’anesthésie gazeux : isoflurane, contrôle d’air et O2 | Confinement A1 et A2
Applications :
- Observation d’échantillons dans l’épaisseur : organoïde, micro-fluidique, tissus et explants (+/-transparisé)
- Imagerie de fluorescence et seconde harmonique
- Acquisitions automatisées dans le temps, multi-position et mosaïque
- Microscopie intravitale avec anesthésie et perfusion.
- Observation à haute résolution en chambre optique : études longitudinales in vivo
Localisation : Campus Institut Pasteur Lille - Animalerie IPL
Contact : Sophie Salomé-Desnoulez | 03.20.87.10.40

![[Translate to English:] Accès à l'un des organismes partenaires : CNRS [Translate to English:] Organisme partenaire](/fileadmin/_processed_/0/1/csm_CNRS_3a745ef80f.png)
![[Translate to English:] Accès à l'un des organismes partenaires : INSERM [Translate to English:] Organisme partenaire](/fileadmin/_processed_/f/f/csm_1200px-Inserm.svg_e391664898.png)
![[Translate to English:] Accès à l'un des organismes partenaires : CHU Lille [Translate to English:] Organisme partenaire](/fileadmin/_processed_/b/b/csm_chu_25c6c9f960.png)
![[Translate to English:] Accès à l'un des organismes partenaires : Institut Pasteur de Lille [Translate to English:] Organisme partenaire](/fileadmin/_processed_/6/1/csm_pasteur_21b9760e1f.png)