Cytometry & Flow Imaging
The BICeL cytometry platform, spread over the Santé and Institut Pasteur de Lille campuses, offers a set of equipment and skills allowing training, advice and analysis in conventional flow cytometry and spectral cytometry, cell sorting and flow imaging.
The platform is open to all local, regional and national academic users as well as to non-academic users from private companies, for example.
Access to and operation of the platform are subject to rules defined in the terms and conditions of access to the BICeL platforms.
Conventionnal Cytometry
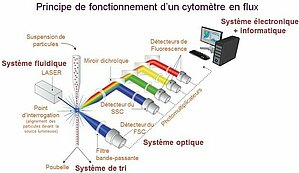
Flow cytometry is a biological technique that allows the precise, quantitative and qualitative multiparametric study of isolated particles in suspension entrained by a liquid flow. Conventional cytometry includes (1) a fluidic system driving cells to one or more excitation sources, (2) a system for collecting the light emitted (fluorescence), (3) digitalization of the signal and (4) analysis software for interpreting the data.
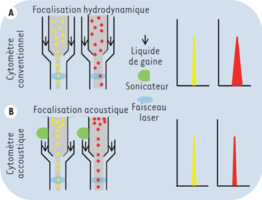
The fluidic system is based on the hydrodynamic focusing of the flow of particles. A pressure slightly greater than that of the sheath liquid is applied to the sample to allow the penetration of the particles into the flow. This strategy favors the alignment of the particles one behind the other at the entrance to the analysis chamber. As the pressure applied to the sample is increased to speed up the speed of analysis, the flow of particles widens. These are then distributed more randomly in the core of the fluid, which affects the homogeneity of the excitation and the collection of signals.
Cytometry is ubiquitous today, as well used in medical analysis laboratories as in research laboratories. It is also found on board ships, or even buoys, for marine biology studies in which the natural pigments of microorganisms are the main criteria for identifying and quantifying populations.
Instruments available:
- Campus Santé
- Campus Pasteur
Cell sorting
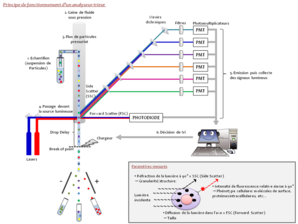
Conventional flow cytometry allows the simultaneous sorting of one or more populations of monodisperse cells during a single analysis.
For this purpose, the liquid stream is electrically charged, then fractionated into a succession of droplets by vibrating the nozzle by a piezoelectric system which will determine the frequency of droplet formation. The droplet containing the desired cell is deflected by passing through an electrostatic field and collected in a collecting vessel. If the cell belongs to an unselected subpopulation or if the droplet formed does not contain cells, the liquid vein will not be charged and the droplet will be eliminated. Cell sorting requires very high stability of the jet formed to guarantee the purity of the populations collected.
It is possible to sort, depending on the devices, from 1 to 6 different populations simultaneously in tubes or to recover the cells directly in culture plates (6, 12, 24, 96 wells, etc.).
Instruments available:
- Campus Santé
- Campus Pasteur
Acoustic Cytometry
In acoustical cytometry, the focusing of the flow is no longer hydrodynamic. Thanks to a sonication probe attached to the inlet of the analysis cuvette, the flow becomes independent of the pressure exerted by the sheath liquid and the particles align with the core of the fluid, then receiving the same excitation by the laser beams. The ultrasound emitted by the sonication probe is similar to that used for ultrasound machines.
This improvement allows much greater cell passage speeds, of the order of 5 to 10 times compared to the hydrodynamic focusing, and without loss of signal quality.
Instrument available:
- Campus Pasteur
Spectral Cytometry
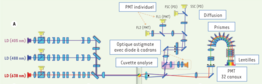
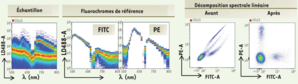
Spectral cytometry allows sampling of the spectrum emitted by a high-resolution fluorochrome. In the analysis chamber, the cells are subjected sequentially to a 488nm laser and then to the combination of two lasers 405 and 638nm. The spectral separation optic is made up of 10 prisms. They spread the fluorescence emitted between 500 and 800nm and a directs towards a series of lenses which concentrate the light on a photomultiplier.
The fluorescence signal of a multibrand sample is then summed up as the sum of the fluorescence of the antibodies coupled to the fluorochromes used and to the background noise coming from the cells themselves. A mathematical linear decomposition algorithm makes it possible to deduce the abundance of each of the probes from the spectral imprint of the multibrand sample and from the reference spectra of the fluorescent molecules used.
Thus, after the acquisition of the samples and then application of the spectral decomposition, the data obtained can be analyzed.
Instrument available:
- Campus Santé
When to use cytometry?
- Multicolor immunophenotyping
- Cell cycle
- Mitochondrial potential, apoptosis, oxidative stress, mitochondrial mass
- Microorganisms
- Sperm
- Absolute count
- Cloning
- Detection, characterization and sorting of rare events such as stem cells
- Miltenyi: Miltenyi Biotec | Biotechnology Company | France
- Biolegend: BioLegend | Your Partner for Antibodies, Proteins, Kits, Proteogenomics, Custom Services, and Reagents in Life Science
- Life Technologies: Life Technologies | Thermo Fisher Scientific - FR
- eBiosciences: eBioscience | Thermo Fisher Scientific - FR
- Sony Biotechnologies : Flow Cytometry Reagents - Reagents (sonybiotechnology.com)
- BD Biosciences: Research Reagents | Conjugated Reagents (bdbiosciences.com)
- Beckman Coulter: Flow Cytometry Reagents, DuraClone IM - Beckman Coulter
- Ozyme: Anticorps et produits liés (ozyme.fr)
- Abcam: Antibodies, Proteins, Kits and Reagents for Life Science | Abcam
- Biorad: Flow Cytometers | Bio-Rad Laboratories
- ThermoFisher Scientific: Fluorescence SpectraViewer | Thermo Fisher Scientific - FR
- BD Biosciences: Spectrum Viewer | BD Biosciences-US
- Miltenyi Biotec: Spectra Viewer | Fluorescence | Tools Miltenyi Biotec | France
- Biolegend : Spectra Analyzer (biolegend.com)
- Fluorofinder: FluoroFinder 2.0
- ThermoFisher Scientific : Flow Cytometry Panel Builder | Thermo Fisher Scientific - FR
- BD Biosciences : BD Horizon™ Guided Panel Solution (GPS) tool | BD Biosciences-US
- Biolegend: Flow Cytometry Tools (biolegend.com)
- Kaluza : Kaluza, Logiciel d’analyse de cytométrie en flux - Beckman Coulter
- FlowJo : Home | FlowJo, LLC
- ModFit : ModFit LT™ (vsh.com)
- CytExpert : Logiciel CytExpert pour la plateforme CytoFLEX - Beckman Coulter
Free trial for 30 days
- OMIQ: https://www.omiq.ai/
The flow core has a site license that can be made available to users (please contact the platform staff for details).
- BD Biosciences : Biosciences | BD Biosciences-EU
- Beckman Coulter : Life Sciences Home - Beckman Coulter
- Sony Biotechnologies : Sony Biotechnology Inc.
- ThermoFisher Scientific : Thermo Fisher Scientific - FR
- CYTEK: Full Spectrum Flow Cytometry Systems | Discover the Advantages | Cytek Biosciences

![[Translate to English:] Accès à l'un des organismes partenaires : CNRS [Translate to English:] Organisme partenaire](/fileadmin/_processed_/0/1/csm_CNRS_3a745ef80f.png)
![[Translate to English:] Accès à l'un des organismes partenaires : INSERM [Translate to English:] Organisme partenaire](/fileadmin/_processed_/f/f/csm_1200px-Inserm.svg_e391664898.png)
![[Translate to English:] Accès à l'un des organismes partenaires : CHU Lille [Translate to English:] Organisme partenaire](/fileadmin/_processed_/b/b/csm_chu_25c6c9f960.png)
![[Translate to English:] Accès à l'un des organismes partenaires : Institut Pasteur de Lille [Translate to English:] Organisme partenaire](/fileadmin/_processed_/6/1/csm_pasteur_21b9760e1f.png)